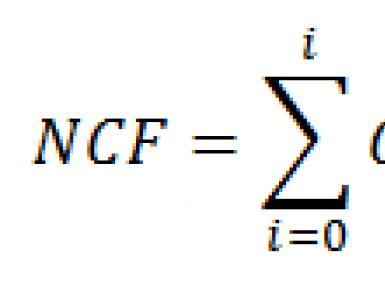Cleaning methods in chemistry. Coursework: basic methods of purification and separation of substances
Reagents produced by industry or obtained in the laboratory may contain insoluble and soluble impurities.
According to the degree of purity, i.e. According to the content of the main substance and permissible impurities, the reagents have an appropriate classification (Table 14). It is indicated on the labels of commercial reagents.
Table 14. Classification of reagents by degree of purity
The first three brands cover all general purpose reagents. Preparations of higher purity are used only for special work, where sometimes even millionths of a percent are unacceptable. They are used in the semiconductor materials industry, radio electronics, and quantum electronics.
When working with reagents, you should always remember that a decrease in impurities even by one order of magnitude, especially starting from 10 -3%, leads to a sharp increase in the price of the substance. Therefore, high-purity preparations cannot be used for low-responsibility work. On the other hand, if required, the purity of the reagent is increased special methods purification, and control the purity of the compound by qualitative and quantitative analysis or determination of its physical characteristics: melting point, boiling point, relative density, refractive index.
In laboratory practice, the following methods for purifying reagents are most often used: recrystallization from solution and sublimation for solids, distillation or rectification for liquids, and sorption of impurities in the case of gases.
In addition, to purify liquids and solutions, sedimentation or coprecipitation of impurities is used (using chemical reagents or electrolysis), as well as extraction and sorption. Metals are purified by recrystallization from the melt, in particular, by zone melting. Let's look at some of the listed methods.
Zone melting. The method of metal purification by zone melting, like purification by crystallization from a melt, is based on the greater solubility of impurities in the melt than in the solid phase of M. With zone melting, the rod of the material being purified slowly moves through a narrow heating zone, melting only in it. In this case, the mixtures, accumulating in the melt, move to the end of the rod. The melting is repeated several times and then the end of the rod, where the impurities have accumulated, is cut off.
Extraction is a method of extracting a substance from one liquid phase to another through the interface between these phases due to the greater solubility of the extracted (extracted) substance in the second liquid. For example, you can purify water from iodine by extracting it with benzene. To create a larger extraction surface area and thus increase the speed of the process, the liquids are vigorously stirred until an emulsion is formed. Then, after settling until the phases are almost completely separated, they are separated (in a separating funnel).
Sorption(from the Latin word “sorbeo”, which means “I absorb”) is the phenomenon of extraction, for example, of a gas from a gas mixture (or a dissolved component from a liquid phase) by a substance in solid state. This substance is called sorbent. Sorption occurs due to the formation of bonds between the atoms of the absorbed compound and the surface atoms of the sorbent. Depending on the type, strength and number of these bonds, particles (molecules, atoms or ions) different substances are retained on the surface of the sorbent with different strength. Therefore, they are absorbed by it to an unequal extent, which makes it possible to separate their mixtures.
For example, you can clear the air of moisture and carbon dioxide using calcium chloride, which practically does not absorb nitrogen and oxygen, but absorbs water and carbon dioxide molecules in significant quantities.
Among the different types of absorption, special emphasis is placed on ion exchange sorption, based on the reversible stoichiometric exchange of solution ions for sorbent ions, which in this case is called ionite.
If an exchange of cations occurs, then the ion exchanger is called cation exchanger, if anions – then anion exchanger. When the cations of the ion exchanger are hydrogen ions, then the cation exchanger is said to be in the H-form and is essentially a poorly soluble polymeric polybasic acid. Similarly, the anion exchanger in the OH form can be considered as a polymeric polyacid base.
If a sodium chloride solution is passed through a column with cation exchanger granules in the H-form, then hydrochloric acid of the appropriate concentration will come out of the column. And after the resulting acid passes through a column with an anion exchanger in the OH form, it turns out pure water. The method is based on this fine water purification using ion exchangers from water-soluble electrolytes.
Recrystallization purification method consists in preparing a saturated solution of a given substance at one temperature and isolating its crystals at another, i.e. it is based on the dependence of s on temperature. This dependence is shown graphically in Figure 7.
According to the solubility curve, for example, of potassium nitrate, we find that from its solution, saturated at 45 0 C, after cooling to 0 0 C, about 60 g of potassium nitrate will precipitate (per 100 g of water). Moreover, if the original salt contained impurities soluble in water, then with the indicated decrease in temperature, saturation with respect to them does not occur, so they will not fall out along with the crystals of the salt being purified, although small amounts of impurities are “captured” by them.
However, repeated recrystallization can obtain an almost pure substance. To reduce the amount of impurities sorbed by the surface of the crystals, they are washed after separation from the mother liquor. (The mother liquor is the solution from which the precipitate was formed.)
Sublimation cleaning method(sublimation) consists of transferring a compound from a solid state to a gaseous state (without the melting stage), and subsequent crystallization of the resulting vapors on a cooled surface. This method can be used to clean highly volatile substances (iodine, benzoic acid, etc.) from non-volatile impurities. To understand the physicochemical essence of sublimation, consider the phase diagram, for example, (Fig. 13).
Each point of the diagram corresponds to a certain state of the system for given p and T, and I is the region of the solid state of the substance, II is the liquid state, III is the gaseous state. Point A, at which the lines separating the phases converge, is called triple, because all 3 phases are in balance in it. For this point corresponds to a saturated vapor pressure of 90 mm Hg. and temperature 116 0 C.
If you move along straight lines 1–4, i.e. above point A, then at point 2 iodine will melt, and at point 3 it will boil.
If we take the state of the system corresponding to point 5 (i.e. below point A), in which the solid phase has a temperature T', and the saturated vapor pressure above it is equal to p', and heat solid iodine at constant p, then the change in the state of the system straight line 5–7 will be reflected. Moreover, at point 6, when the saturated vapor pressure is equal to external p, the process will begin intense sublimation. (Segment 6–7, like 3–4, corresponds to heating vapors in the absence of its other phases.)
However, this all applies to equilibrium states. And under nonequilibrium conditions, sublimation of iodine is possible if the pressure of its saturated vapor is at least less external pressure, but quite high. At the same time, on initial stage heating solid iodine below, than at point A, and will remain so if the process is carried out at open vessel, because vapors are provided with free escape from the system, which is actually sublimation under non-equilibrium conditions.
If you heat iodine, for example, in a test tube covered with cotton wool, then its vapors, being heavier, will displace air from the vessel (through the cotton wool). Therefore, it will increase, and when it becomes above 90 mm Hg. (at T, providing a liquid state), it will melt. That's how they get it liquid iodine.
Purification of a substance by distillation or distillation based on the transformation of liquid into steam followed by its condensation. This method separates a liquid from non-volatile solid impurities dissolved in it. For example, using distillation, natural water is purified from the salts it contains. The result is the so-called distilled water.
Gas purification. The gases obtained in the reactions are usually contaminated with water vapor and impurities of other volatile substances. The gas is purified by passing it through compounds that absorb these impurities. Liquid or solid substances are used as an absorber, with liquids placed in a Drexel flask, and solids (in the form of granules) in a calcium chloride tube or Tishchenko flask (Fig. 14).
The choice of gas purification method depends on the physical and chemical properties of not only the gas itself, but also the impurities. For example, carbon dioxide obtained in the Kip apparatus contains a small amount of hydrochloric acid and water vapor released from the HCl solution. This gas is passed first through a wash with water (to absorb HCl), and then through a calcium chloride tube (water vapor is sorbed). Etc. The carbon dioxide is almost pure.
Close the hole of the Wurtz flask with a stopper with a thermometer (2), attach a refrigerator (3), an allonge (4), lower the latter into the receiver (5). On the stove (6) through an asbestos mesh, heat the solution in the flask to a boil. At what T will it boil? Does the boiling point change during liquid evaporation?Finish heating when 100–120 ml of liquid has collected in the receiver. Measure its density. Does it contain copper sulfate? How to install it?
2. Purification of iodine by sublimation. Place 0.3 g of crystalline iodine and 0.1 g of potassium iodide in a sublimation glass (to remove Cl 2 and Br 2 impurities contained in iodine), and stir with a glass rod. Cover the glass with a round-bottomed flask of cold water and carefully heat it through an asbestos mesh (Table 6). After the release of vapor (what color?) has stopped, separate the crystals from the flask, weigh them and determine the percentage of iodine yield.
3. Purification of copper sulfate pentahydrate by recrystallization. Calculate the amount of water and required to prepare a solution saturated at 60 0 C, so that when it is subsequently cooled to 0 0 C, 7 g of crystalline hydrate is released, using the following data:
| T 0 C | |||||||||
| S, g/100 g H 2 O | 12.9 | 14.8 | 17.2 | 20.0 | 22.8 | 25.1 | 28.1 | 34.9 | 42.4 |
Typically, pentahydrate contains impurities of potassium chloride, as well as sand and pieces of coal. Therefore, for cleaning, weigh out the original salt by 10% more than the calculated mass. Measure the required volume of water with a cylinder, pour it into a 50 ml glass, boil the water and dissolve a portion of the salt to be purified in it while stirring.
Make sure there are chloride ions in the prepared solution. To do this, to 3 drops of it add a drop of AgNO 3 solution and two drops nitric acid. What is being observed? Why? Then filter the copper sulfate solution heated to a boil through a pleated filter prepared in advance.
While stirring the filtrate with a glass rod, cool it to room temperature, and then to 0 0 C in a crystallizer with water and ice. Separate the precipitated crystals from the mother solution by filtration and wash them (why?) with 5-10 ml of cold distilled water. Test the purified salt solution, mother liquor, and wash water for chloride ions and draw conclusions.
Then remove the salt crystals from the funnel and squeeze them between sheets of filter paper until they no longer stick to the dry glass rod. Weigh the resulting salt on a technical chemical balance. Estimate the mass of salt as a percentage relative to the original sample. What explains the relatively low yield of the product purified by recrystallization?
4. Carbon dioxide purification. Fill the Wurtz flask 1/5 of its volume with pieces of marble, attach a gas outlet tube to it, add 30 ml of a 20% HCl solution and immediately close the flask with a stopper. What is being observed? How can the resulting carbon dioxide be contaminated?
Pass the evolved gas for 10–15 minutes through a Drexel flask with distilled water and a calcium chloride tube filled with anhydrous copper sulfate connected in series with it. (How does its color change? Why?). Test the contents of the wash bottle for the presence of Cl – and H + ions, using AgNO 3 solution and indicator paper, respectively. Draw conclusions.
1. PURPOSE OF THE WORK
Goal of the work– familiarization with the basic techniques of working in an organic chemistry laboratory, laboratory instruments and glassware, methods for isolating and purifying organic substances.
2. THEORETICAL INTRODUCTION
METHODS FOR PURIFYING ORGANIC SUBSTANCES
Filtration
Filtration is carried out to separate the precipitate from the liquid phase when separating substances, purifying them, washing the precipitate, etc.
To separate solid particles from liquid, in the simplest case, the liquid is drained from the sediment (decantation method); in other cases, filtration is used through a funnel with a filter. Filtration efficiency depends on the porosity of the filter as well as the pressure drop on either side of the filter. Filters are most often made from various types of filter paper, fiberglass, porous glass and fluoroplastic.
For simple filtering, use a funnel with a pleated filter.
More effective filtration is carried out under vacuum, for which two types of filter funnels are usually used: “Schott funnels” with a porous glass plate and a Buchner funnel equipped with a well-fitted paper filter, connected to a Bunsen flask.
The paper filter is pre-wetted on the funnel with a solvent, which is then sucked off. After this, the solution with crystals is transferred to a paper filter. Suction of the uterine fluid is ensured by a water-jet pump connected to a Bunsen flask through a safety bottle. The required filtration speed is achieved by adjusting the water jet in the water-jet pump, which creates a reduced pressure in the Bunsen flask.
To remove residual mother liquor, wet crystals are washed with several portions of a minimal amount of solvent while gently stirring the crystals. Sometimes the filter cake is only saturated with solvent, and then a vacuum is turned on to suck it out.
The crystals on the filter are squeezed out of the solvent with the flat side of a glass stopper, then the precipitate is sent for drying.
Drying
Drying is the process of freeing a substance in any state of aggregation from impurities of any liquid, most often water, as a solvent.
Drying of liquids is carried out using substances that can absorb water - desiccants. In this case, desiccants should not interact with the substance being dried and the solvent, dissolve in them, or cause oxidation, polymerization or other undesirable processes. The dryer must be as efficient as possible, i.e., ensure the fastest and most complete removal of liquid impurities from the system.
The list of substances used as desiccants for organic liquids and their intended purpose is given in Table 1.1. To carry out drying, the organic solution is shaken with a small amount of desiccant (up to 3% by weight of the solution), and the resulting aqueous solution of the desiccant is drained. The process is repeated until the desiccant crystals stop spreading in the organic solution.
Drying of solids from volatile impurities is carried out in air or at optimal temperature in the drying cabinet. For drying in a vacuum, vacuum desiccators are used; hygroscopic compounds are usually dried in this way.
Table 1.1 - Drying agents for organic liquids and solutions
Dehumidifier | What can be dried | What can't be dried |
Hydrocarbons, their halogen derivatives, ethers and esters, aldehydes, ketones, nitro compounds and solutions of substances sensitive to various influences | ||
Hydrocarbons and their halogen derivatives, ethers, nitro compounds | Alcohols, phenols, aldehydes, ketones, acids, amines, amides, esters |
|
Amines, ketones, alcohols | Substances with acidic properties |
|
Amines, ethers, hydrocarbons | Aldehydes, ketones, acids |
|
Hydrocarbons, ethers, tertiary amines | Halogenated hydrocarbons, alcohols, acids (Danger of explosion!) |
|
Н2SO4 (conc.) | Neutral and acidic substances | Unsaturated hydrocarbons, alcohols, ketones, bases |
Hydrocarbons and their halogen derivatives, acid solutions | Bases, alcohols, ethers |
|
Molecular sieves (aluminosilicates Na, Ca) | Used for drying solvents. Regenerated by heating in vacuum at 150-300°C | Unsaturated hydrocarbons |
Recrystallization

A device for recrystallizing small quantities of a substance.1 - glassWithboiling solvent; 2 - funnel; 3 - pleated filter; 4 - suction tube; 5 - glass “nail”; 6 - filter.
Recrystallization is the simplest method for separating and purifying solids.
The crystallization method consists of the following stages: dissolution of the solid in a minimum volume of boiling solvent (preparation of a saturated solution); filtering the hot solution to remove insoluble impurities (if present); cooling the solution to form crystals; filtering the crystals from the mother liquor and drying them.
The correct choice of solvent is extremely important for successful crystallization. In the solvent, the substance to be purified should dissolve easily when heated and practically not dissolve in the cold, and impurities should also dissolve well in it. The general pattern of solubility is "like dissolves into similar" , i.e., polar compounds are more soluble in polar solvents than in non-polar ones, and vice versa.
After hot filtration, the saturated solution is slowly cooled to room temperature and then placed in a refrigerator to form crystals. Often, to speed up the crystallization process, a sharp-edged glass rod is rubbed along the inner wall of the flask at the liquid level, which leads to the formation of irregularities on the glass surface, which serve as centers for crystal growth. After cooling, the resulting crystals are separated from the mother liquor by filtration, washed and dried.
Sublimation

Sublimation device: 1-hour glass; 2- glass; 3 - thermometer; 4-sand bath.
Sublimation involves the evaporation of a substance when heated below its melting point, followed by condensation of the vapor on a cooled surface. Purification of a solid by sublimation is possible only if its vapor pressure is higher than the vapor pressure of impurities. When the vapor pressure of the solid matches the applied pressure, the best results are obtained. For example, stilbene is sublimated at a temperature of 100°C and a pressure of 20 mm Hg. Art.
Sublimation is carried out in a vacuum in a sublimator device or at atmospheric pressure in a porcelain cup, closed on top with a filter with numerous holes pierced with a needle and a glass funnel. Before sublimation, solvents and other volatile products are removed from the substance to be purified to avoid contamination of the sublimate.
Distillation
volatile" solvents with a boiling point of up to 100°C at a bath temperature of 50-60°C.
The simplest distillation is effective only if the components of the mixture to be separated differ in boiling points by at least 60°C. In all other cases, the substances are subjected to fractional distillation using different types distillation columns (distillation). The simplest column can be a hollow tube or a Vigre herringbone reflux condenser.
At atmospheric pressure, substances with boiling points from 40°C to 180°C are usually distilled; liquids with a boiling point less than 40°C are distilled with large losses. With more high temperature boiling, there is a danger of thermal decomposition of the substance, and it is distilled in a vacuum, since with a decrease in pressure the boiling point decreases.
Extraction

Extraction device: 1 - separating funnel; 2 - liquid with higher density; 3 - liquid with lower density; 4 - plug, 5 - foot, 6 and 7 - receivers.
Extraction is a method of extracting one or more components of a mixture or separating them by transferring them from one phase to another.
Solid-phase extraction (extraction) consists of extracting organic compounds from solids by treatment with an organic solvent - extractant; in liquid-phase extraction, one phase is, as a rule, an aqueous solution, the other - an organic one. The extractant must have minimal solubility in water and be selective with respect to the extracted substance.
Typically, extraction is carried out from the aqueous (neutral, acidic, basic) phase with a solvent that is immiscible with water (for example, dichloromethane, chloroform, ethers, etc.). In the case of polar products (for example, alcohols, carboxylic acids, amines), the aqueous phase is saturated with sodium chloride (salting out) before extraction.
METHODS FOR IDENTIFYING ORGANIC COMPOUNDS
Determination of the refractive index of a liquid
The refractive index of a substance is one of the most important physical constants and is used to identify substances and test their purity. The refractive index is determined by the nature of the substance and the wavelength of the incident light and is a constant value for a given substance. Most often, the refractive index is determined at 20°C for the sodium D line (589 nm), which is reflected by the designation nD. For liquid organic substances, the refractive index decreases with increasing temperature and usually ranges from 1.3 to 1.8.
When a ray of light falls on the interface between two transparent homogeneous media, part of it is reflected at an angle equal to the angle of incidence a, and part is refracted at an angle b. According to the law of refraction, the ratio of the sine of the angle of incidence to the sine of the angle of refraction is a constant value called the relative index (or coefficient) of refraction of the second substance in relation to the first:
Refractometers are used to determine the refractive index.
Differential" href="/text/category/differentcial/" rel="bookmark">differential thermal analysis (DTA) or differential scanning calorimetry (DSC).
Reference literature" href="/text/category/spravochnaya_literatura/" rel="bookmark">reference literature data on the solubility of the specified substance in a given solvent at room temperature and heating, calculate the volume of solvent required for recrystallization of 2 g of a contaminated sample. Leave 0.1 g sample to determine the melting point.
2. Place the sample in a beaker, add the calculated amount of solvent and heat until the solid phase is completely dissolved with stirring. Next, the glass is removed from the stove, the contents are cooled to room temperature on the workbench, and, if necessary, in the refrigerator.
3. The precipitate that forms is separated by filtration through a paper filter, then the filter with the precipitate is dried in air.
4. Collect crystals from the filter onto a pre-weighed watch glass, dry them in an oven and weigh them.
Experiment 2. Purification of a substance by sublimation.
1. Receive the contaminated substance (naphthalene, benzoic acid) from the teacher and weigh it. Leave 0.1 g of the starting material to determine the melting point. Find the melting point of a pure substance using a reference book.
2. A small porcelain cup is covered with a sheet of filter paper with small punctures (20-30 holes) and the filter paper is pressed tightly with an overturned glass funnel, the hole of which is covered with cotton wool.
3. The porcelain cup with the sample is placed on an electric stove and carefully heated to a temperature below its melting point by 10-20°C. Heating is carried out until crystals form on the surface of the glass funnel.
4. Stop heating the installation, carefully cool it, collect the crystals and weigh them. The melting temperatures of the samples before and after recrystallization are determined. Compare the obtained data with the reference data.
PROCESSING OF EXPERIMENTAL RESULTS1. The laboratory journal provides theoretical information on this topic.
2. Record the progress of experiments 1 and 2.
3. Write out reference data and make the necessary calculations.
4. The results are placed in table 1.2.
Table 1.2 - Summary table of experimental results.
Substance, name, chemical formula | Constants (reference data): density, Tmel | Mass of contaminated substance, g | Mass of substance after purification, g | Solvent volume, ml | Sublimation or melting point, °C | ||
| safety precautions that should be followed when working with organic substances. |
Purpose of the lesson: To become familiar with the basic methods of purifying substances, in particular, with filtration under normal pressure (simple and folded filter), hot, under vacuum.
Lesson plan:
1. Strengthen knowledge and skills on basic methods of purification of substances.
2. As instructed by the teacher, purify the contaminated salt using the filtration method.
Materials and equipment: beakers, glass rods, flat-bottomed and conical flasks, funnels, tripod, filter paper, solution table salt, sand.
Laboratory workshop
For purification of substances, depending on their state of aggregation, they are used various methods. Purification of solids is usually carried out by two methods: recrystallization and sublimation, liquids - by filtration and distillation, gases - by absorption of impurities with various chemical reagents.
Filtration is used to separate (purify) liquids from insoluble solids. Filtration is carried out by passing liquid through porous materials - filters.
Quartz sand, asbestos, glass wool, porcelain plates (Gooch crucibles), pressed glass (Schott crucibles), textile fabrics, cotton wool, paper filters (filter paper of various sizes) can be used as filter materials.
The choice of filter material depends on the properties of the filtered liquid and the size of solid particles. In the laboratory, paper is most often used.
filters - simple or folding. A simple filter is used when sediment is needed for further work. A simple filter is prepared from a square sheet of paper corresponding to the size of the crow, fold it in half (Fig. 33), as shown by the dotted line, and in half again
The outer corners are cut in an arc so that the edge of the filter is 0.5-1 cm below the edge of the funnel. Unscrew one fourth of the folded filter and insert it into
funnel, press it with your fingers to the walls of the funnel, moistening it with distilled water. It is necessary that the filter fits tightly to the funnel frames.
Pleat filter. Please read carefully how to make a pleated filter. Test your skills in making a pleated filter with your teacher.
For easy-to-filter liquids, filtration under normal pressure is used; for difficult-to-filter liquids, vacuum filtration is used. For viscous liquids and saturated solutions, hot filtration.
For filtration under normal pressure, assemble the device. When a little liquid remains, the sediment is shaken and transferred to the filter. The liquid that passes through the filter is called the filtrate or mother liquor. The remaining sediment is washed off onto the filter with distilled water from the washer.
Washing the sediments is done with water or a special solvent, pouring it in small portions, allowing the solution to drain completely and only then pouring the next portion. After 4-5 washes, the completeness of washing from certain impurities is checked. To do this, a few drops of the flowing liquid are taken into a clean test tube and a reaction is carried out on the ion being washed off (for example, Cl ion - AgNO 3; SO 4 ion - BaCl 2). The appearance of turbidity requires further washing of the sediment. The washing liquid is collected separately from the main filtrate.
The decanting method is used to separate and wash poorly soluble and slowly filterable sediments. Before filtration begins, the resulting precipitate is allowed to settle to the bottom of the vessel. The clarified solution is carefully poured from the sediment onto the filter. The solvent is again added to the precipitate, stirred, and the solution is allowed to settle. The liquid is drained again, and a solvent is added to the precipitate and this is repeated several times. The precipitate is then transferred to a filter for further washing.
Exercise. Assemble the device for filtering under normal pressure. Familiarize yourself with the tripod and its assembly. Filter according to teacher's instructions 50 ml
suspended matter - sand - water, clay - water. Master the methods of quantitative sediment transfer using a stick and a wash.
To more quickly separate solids from liquids, vacuum filtration is used. Filtration under reduced pressure is carried out in a device that consists of a thick-walled Bunsen flask (1) with a side extension and a porcelain Buchner funnel (2) with a grid bottom inserted into it using a rubber stopper. Place two filters at the bottom of the funnel, one along the diameter of the bottom of the funnel, and the other at 0.5 cm more than the first. Having cut along the contour of the funnel, the filter is finally adjusted to the funnel. The smaller filter is placed at the bottom of the funnel, moistened with water and pressed against the bottom of the funnel, and a second filter is placed on top, the edges of which are straightened along the walls of the funnel. The vacuum is created using a pump. The device is connected to the pump in order to
the filters are firmly attached to the bottom and walls of the funnel, then the device is turned off. Using a glass rod, pour the solution with the sediment into a Buchner funnel, after which the device is connected to the pump through a safety bottle. The vacuum in the flask should be created gradually as sediment accumulates. The sediment on the filter should be squeezed out.
After completing the filtering, the flask should be disconnected from the safety flask and only then close the water tap.
To remove the precipitate from the funnel, remove it from the flask, turn it over onto a sheet of filter paper and, by striking the funnel with your hand, remove the precipitate. Instead of a Buchner funnel, for the same purposes you can use Gooch crucibles or glass Schott funnels with different pore diameters.
Pawing As instructed by the teacher, assemble the device with a Buchner funnel and a glass Schott funnel. Familiarize yourself with the operation of a water jet or other pump.
Questions and tasks
1. What is filtering used for?
2. Why are plain and pleated filters used?
3. Name the materials from which the filters are made?
4. Filtration technique at normal pressure.
5. Vacuum filtration technique.
6. Abstract topics
7. Experiments proving the complexity of the structure of the atom.
8. Attempts to systematize elements. Discovery of the periodic law.
Tasks and exercises for SRS
N.L.Glinka Problems and exercises in general chemistry. 140-164 tasks and questions. Pages 37-39.
Laboratory work No. 3
Subject: Basic techniques for working in a chemical laboratory. Scales. Weighing
Purpose of the lesson: to master the basic techniques of working in a chemical laboratory and to master weighing techniques. To become familiar with various types scales
Lesson plan:
1. Familiarize yourself with the work of technical, technochemical, analytical, and electronic scales.
2. As instructed by the teacher, weigh the required amount of the substance.
Materials and equipment: technical scales, technochemical balances, analytical balances, electronic scales, weights.
Laboratory workshop
Weighing on a lever scale is the comparison of the mass of a given body with the mass of weights, the mass of which is known and expressed in certain units (mg, g, kg, etc.). Scales are the most important instrument in a chemical laboratory, since not a single work in it is complete without determining the mass of a particular substance or container in which the substance being weighed will be placed.
Techno-chemical balances are used to weigh substances with an accuracy of 0.01 g (Fig. 1)

Rice. 1. Techno-chemical scales and weights (1 - column, 2-arrangement, 3 - scale pans, 4 - arrow, 5 scale, 6 plumb line, 7 - screws for installing the scales in a horizontal position, 8 - rocker arm, 9 - screws for balancing empty scales)
The design principle of techno-chemical and analytical balances is the same. There are three prisms on the metal rocker (equal-arm lever): two at the ends and one in the middle (Fig. 2). The middle prism rests on a plate located on the central column of the scales and which is the fulcrum. In analytical balances the plate is made of agate. On the side prisms there are plates from which the scales are suspended. The rocker arm is equipped with a long arrow, which shows on the scale the amount of deviation of the rocker arm from the horizontal position. When the rocker is in a horizontal position, the needle is at the zero scale mark.
Before weighing, the scale must be plumb. It is not permitted to move or move the scale after installation. Before you start weighing, you need to check the scale. To do this, by smoothly turning the screw that raises and lowers the rocker arm (lock), the scales are brought into working position and the arrow is observed to swing in either direction from the middle division of the scale located at the bottom of the scales. If the arrow deviates from the center line of the scale by an equal number of divisions in both directions, or in one direction by 1-2 divisions more than in the other, then the scales can be considered suitable for operation. At the end of the test, the scales must be locked, that is, moved to the non-working position by turning the lock back.
When weighing, the following rules must be observed:
You can place objects and weights on the scales, remove them from there, or touch the working part of the scales with anything only after the scales are completely locked.
Do not place hot, wet or dirty objects on the scale pan. When working with liquids, never allow liquid to come into contact with scales or weighing scales.
Place the item to be weighed on the left pan of the scale, and the weights on the right.
Do not place the substance to be weighed directly on the scale pan. Weigh solids on watch glasses (concave glasses), in bottles, in crucibles or on pieces of glossy paper.
Take weights only with tweezers and when removing them from the scales, place them in the slots from which they were taken. Under no circumstances should weights be placed on the table.
First, you need to take a weight that approximately corresponds to the weight of the object. If the weight turns out to be greater than necessary, then you need to take the next one, etc., until balance is achieved, i.e. approximately the same deviation of the needle in both directions from the middle of the scale as it was before weighing.
Having calculated the total weight of the weights, write it down in workbook. Do not write down the weighed amount on separate sheets or scraps of paper.
Do not take weights from another set of weights.
When sequentially weighing one or various items which are carried out in connection with the same work, the same scales and weights should be used.
After weighing, be sure to lock the scales. Do not leave anything on the awnings.
Every weighing is inevitably accompanied by an error. Therefore, in order to find a weight that is as close as possible to the true one, it is necessary to perform 4-5 weighings. During consecutive weighings, do not remove the item from the scale each time. One weighing is separated from another only by adjusting the scales.
The error allowed during weighing can be expressed as a mean square error. The calculation of the mean square error is carried out as follows. Let's assume that 1,2,3... weighings are performed and the following results are obtained:
a 1, a 2,.. a p
find the arithmetic mean of these values

The root mean square error 6 is given by the following expression
Thus, the weight of the object is: A = a ± 6
Task: Weigh on a technical-chemical balance two small objects taken from a laboratory assistant (weighing from 1 to 100 g), with an accuracy of 0.01 g. Determine the mean square error of the weighings.
Questions and tasks
1. General rules work in a chemical laboratory.
2. Device of scales. Scale accuracy. Weighing technique.
3. Errors when weighing. Root mean square weighing error.
Tasks and exercises for SRS
N.L.Glinka Problems and exercises in general chemistry. L" 99-114 tasks and questions. Pages 26-27.
Laboratory work No. 4
Topic: Sublimation.
Purpose of the lesson: To become familiar with the methods of purification of substances: sublimation, distillation, recrystallization.
Materials and equipment: round-bottomed flasks, beakers, funnels, tripod, burner, mortar, porcelain cup, iodine.
Laboratory workshop
Under normal conditions, iodine is a solid substance with a molecular crystal lattice. When molecules evaporate from the surface of a solid, this is called sublimation. Both evaporation and sublimation produce vapors. Violet smoke is iodine vapor; before our eyes, with slight heating, iodine sublimes: a transition from a solid to a gaseous state, bypassing the liquid state. Iodine vapor rises and settles on the cooler walls of the test tube in its upper part. Here solid iodine is formed again. Solid iodine becomes liquid at 113°C, liquid iodine boils at 184°C.
Assignment: As directed by the teacher, add 2 hours of CaO and 1 hour of KI to 6 parts by mass of technical I 2, grind the mixture in a mortar. Technical iodine is placed at the bottom of the glass to be cleaned. The glass is covered with a round-bottomed flask filled with cold water, placed in a sand bath and the heating is turned on.
Laboratory work No. 5
Introduction
Boron is mainly used in the form of borax.
BOROX - sodium salt of tetraboric acid. It is widely used in the production of fusible glaze for earthenware and porcelain products and, especially for cast iron cookware (enamel); In addition, it is used for preparing special types of glass.
The use of borax in soldering metals is based on the dissolution of metal oxides. Since you can only solder clean surfaces metals, then to remove oxides, sprinkle the soldering area with borax, put solder on it and heat it. Borax dissolves oxides, and the solder adheres well to the metal surface.
Boron plays an important role in plant life. the presence of a small amount of boron compounds in the soil is necessary for the normal growth of agricultural crops, such as cotton, tobacco, sugar cane, etc.
In nuclear engineering, boron and its alloys, as well as boron carbide, are used for the manufacture of reactor rods. Boron and its compounds are used as materials that protect against neutron radiation.
This work is devoted to methods for purifying borax as the main substance - a source of boron.
Borax and its properties
Sodium tetraborate (“borax”) - Na 2 B 4 O 7, a salt of a weak boric acid and a strong base, a common boron compound, has several crystalline hydrates, and is widely used in technology.
Chemistry
Structure of anion 2− in borax
The term “borax” is used in relation to several related substances: it can exist in anhydrous form, in nature it is more often found in the form of pentahydrate or decahydrate crystalline hydrate:
Anhydrous borax (Na 2 B 4 O 7)
Pentahydrate (Na 2 B 4 O 7 5H 2 O)
Decahydrate (Na 2 B 4 O 7 10H 2 O)
However, the word borax most often refers to the compound Na 2 B 4 O 7 10H 2 O.
Natural springs

Borax, "cottonball"
Sodium tetraborate (Borax) is found in salt deposits formed by the evaporation of seasonal lakes.
Borax (sodium tetraborate decahydrate, Na 2 B 4 O 7 · 10H 2 O) are transparent crystals that completely lose water when heated to 400°C.
Ordinary borax (hydrate decahydrate) forms large, colorless, transparent prismatic crystals; base-centered monoclinic lattice, a = 12.19 Å, b = 10.74 Å, c = 11.89 Å, ß = 106°35´; density 1.69 - 1.72 g/cm3; In dry air, the crystals erode from the surface and become cloudy.
Borax hydrolyzes in water, its aqueous solution has an alkaline reaction.
With the oxides of many metals, borax, when heated, forms colored compounds - borates (“borax pearls”). Occurs in nature as the mineral tincal.
Tinkal, or “Borax” (sodium tetraborate decahydrate, Na 2 B 4 O 7 · 10H 2 O) is a mineral of the monoclinic system, prismatic. “Tinkal” is a word of Sanskrit origin, which is synonymous with the more commonly used name for the mineral - “Borax” (from the Arabic “burak” - white).
White color, glass luster, Mohs hardness 2 - 2.5.
Density 1.71.
Cleavage is average in (100) and (110).
It forms short-prismatic crystals, shaped like pyroxene crystals, as well as solid granular masses and veinlets in clayey rocks.
A typical evaporite mineral.
In air it collapses, losing crystallization water and becomes covered with a crust of tincalconite or kernite, over time turning into them completely.
The so-called Jewelry Borax is sodium tetraborate pentahydrate Na 2 B 4 O 7 5H 2 O.
Borax is used:
· in the production of enamels, glazes, optical and colored glasses;
· when soldering and melting as a flux;
· in the paper and pharmaceutical industries;
· in the production of building materials as an antiseptic component for the production of cellulose insulation “Ekovata”
· as a disinfectant and preservative;
· in analytical chemistry:
o as a standard substance for determining the concentration of acid solutions;
o for the qualitative determination of metal oxides (by the color of pearls);
· in photography - in the composition of slow-acting developers as a weak accelerating substance;
· as a component of detergents;
· as a component of cosmetics;
· as a raw material for boron production;
· as an insecticide in poisoned baits to kill cockroaches.
In dry air, the crystals erode from the surface and become cloudy. When heated to 80°C, the decahydrate loses 8 water molecules; at 100 degrees, slowly, and at 200°C, another water molecule is quickly split off; in the range of 350 - 400°C, complete dehydration occurs.
Solubility of borax (in anhydrous salt per 100 g of water): 1.6 (10°C), 3.9 (30°C), 10.5 (50°C). The saturated solution boils at 105°C.
Borax hydrolyzes in water, so its solution has an alkaline reaction.
The alkaline reaction of the sodium tetraborate solution is due to the fact that a hydrolysis reaction occurs in an aqueous solution with the formation of boric acid B(OH) 3 in the solution:
Na 2 B 4 O 7 = 2Na + + B 4 O 7 2– ;
B 4 O 7 2– + 7H 2 O 2OH – + 4B(OH) 3,
and the release of ammonia upon interaction with NH4Cl corresponds to the equation:
Na 2 B 4 O 7 + 2NH 4 Cl + H 2 O = 2NH 3 + 2NaCl + 4B(OH) 3
Borax dissolves in alcohol and glycerin.
Completely decomposes with strong acids:
Na 2 B 4 O 7 + H 2 SO 4 + 5H 2 O = Na 2 SO 4 + 4H 3 BO 3.
This is exactly how the Dutch alchemist Wilhelm Gomberg, by heating borax with sulfuric acid H 2 SO 4, isolated boric acid B(OH) 3.
With the oxides of some metals, borax produces colored borates (“borax pearls”):
Na 2 B 4 O 7 + CoO = 2NaBO 2 + Co(BO 2) 2,
which is used in analytical chemistry to discover these metals.
When a solution of ordinary borax is slowly cooled at 79°C, octahedral borax Na 2 B 4 O 7 begins to crystallize. 5H 2 O (or “jewelry borax”), density 1.815 g/cm 3, stable in the range 60 - 150 ° C. The solubility of this borax is 22 g in 100 g of water at 65°C, 31.4 at 80°C and 52.3 at 100°C.
Borax is the most important flux that facilitates the smelting process. When cooled, molten borax forms a glaze on the walls of the crucible, protects the melt from oxygen and dissolves metal oxides.
With the slow thermal dehydration of ordinary borax, a pyroborax with a density of 2.371 g/cm 3 and a melting point of 741 ° C is obtained. Borax melts and breaks down into sodium metaborate and boron trioxide, which mix in a liquid state:
Na 2 B 4 O 7 → 2NaBO 2 + B 2 O 3 .
Boron oxide, combining with metal oxides, forms metaborates in the same way as boric acid. Sodium metaborate easily mixes with newly formed metaborates and quickly removes them from the molten metal zone, and new active boron oxide molecules take their place.
Borax has a greater ability to dissolve oxides than boric acid, and is used not only as a melting reducing flux, but also as the most important flux for brazing.
Ordinary borax is obtained from boric acid, from tincal, kernite and some other minerals (by recrystallization), as well as from salt lake water (by fractionated crystallization).
Borax is widely used in the preparation of enamels, glazes, in the production of optical and colored glasses, in welding, cutting and soldering of metals, in metallurgy, electroplating, dyeing, paper, pharmaceutical, leather production, as a disinfectant and preservative and fertilizer.
Purification of substances by recrystallization
Recrystallization is a method of purifying a substance based on the difference in solubility of a substance in a solvent at different temperatures (usually the temperature range from room temperature to the boiling point of the solvent, if the solvent is water, or to some higher temperature).
Recrystallization implies poor solubility of a substance in a solvent at low temperatures, and good solubility at high temperatures. When the flask is heated, the substance dissolves. After the stage of adsorption of impurities (if necessary) with activated carbon, hot filtration (if necessary) and cooling, a supersaturated solution is formed, from which the dissolved substance precipitates. After passing the mixture through a Bunsen flask and a Buchner funnel or centrifugation, we obtain a purified solute.
· Advantage of the method: high degree of purification.
· Disadvantage of the method: strong losses of substance during recrystallization: always part of the dissolved substance will not precipitate, losses during recrystallization often amount to 40-50%.
The solvent can be water, acetic acid, ethanol (95%), methanol, acetone, hexane, pentane - depending on conditions.
If the solvent is water, then heating is carried out in a water bath. Cooling of the supersaturated solution is carried out using a water cooler if the boiling point of the solvent is below 130 degrees, if higher - using an air cooler.
The solubility of most solids increases with increasing temperature. If you prepare a hot, concentrated (almost saturated) solution of such a substance, then when this solution is cooled, crystals will begin to precipitate, since the solubility of the substance is less at a lower temperature. The formation of a cold saturated solution, the concentration of which is less than the initial (hot) one, will be accompanied by crystallization of the “excess” substance.
Dissolution of a substance containing soluble impurities in hot water, and then precipitating it from solution with sufficient cooling is a method of purifying a substance from soluble impurities, which is called recrystallization. In this case, impurities, as a rule, remain in the solution, since they are present there in negligible (“trace”) quantities and upon cooling cannot form their saturated solution.
Some part of the substance being purified also remains in a cold saturated solution, which in laboratory practice is called uterine, and such inevitable (planned) losses of a substance can be calculated from the solubility of the substance at this temperature.
The more the solubility of a substance decreases when the solution is cooled, the higher the yield of recrystallized substance will be.
Many solids form crystalline hydrates when crystallized from an aqueous solution; for example, from an aqueous solution, copper (II) sulfate crystallizes in the form of CuSO 4 · 5 H 2 O. In this case, the calculation must take into account the water that is part of the crystalline hydrate.
Recrystallization has great importance in chemistry and chemical technology, since the vast majority of solids - chemical products, reagents, chemicals, drugs, etc. are obtained from aqueous and non-aqueous solutions, and the final stage of this preparation is crystallization (or recrystallization in order to increase the purity of the product). Therefore, it is very important to carry out these processes efficiently, with minimal losses and high performance quality.
To carry out recrystallization, special chemical glassware and laboratory equipment are used.
The recrystallization process is carried out in several stages:
Choice of solvent;
Preparation of a saturated hot solution;
- “Hot” filtration;
Cooling the solution;
Separation of formed crystals;
Washing the crystals with a clean solvent;
Drying.
Solvent selection
Right choice solvent - a condition during recrystallization.
There are a number of requirements for the solvent:
A significant difference between the solubility of a substance in a particular solvent at room temperature and when heated;
The solvent should dissolve only the substance when heated and not dissolve impurities. The efficiency of recrystallization increases with increasing difference in solubility of the substance and impurities;
The solvent must be indifferent to both the substance and impurities;
The boiling point of the solvent must be 10 - 15°C lower than the melting point of the substance, otherwise when the solution is cooled, the substance will not be released in crystalline form, but in the form of an oil.
Experimentally, the solvent is chosen as follows: a small sample of the substance is placed in a test tube, adding a few drops of solvent to it. If a substance dissolves without heating, such a solvent is not suitable for recrystallization.
The choice of solvent is considered correct if the substance dissolves poorly in it without heating, well - when boiling, and when the hot solution is cooled, its crystallization occurs.
Water, alcohols, benzene, toluene, acetone, chloroform and other organic solvents or mixtures thereof are used as solvents for recrystallization.
The substance for recrystallization is placed in a flask (1), a small portion of the solvent is added and heated under reflux (2) until the solution boils. If the initial amount of solvent is not enough to completely dissolve the substance, the solvent is added in small portions using a funnel through a reflux condenser.
Effective purification of heavily contaminated substances is possible using various adsorbents (activated carbon, silica gel, etc.). In this case, prepare a hot saturated solution of the substance, cool it to 40 - 50 ° C, add an adsorbent (0.5 - 2% by weight of the substance) and reflux it again for several minutes.
"Hot" filtration
To separate mechanical impurities and adsorbent, the hot solution is filtered. To prevent the release of substances on the filter, various methods are used.
A simple “hot” filtration installation (Fig. 3.2) consists of a special “hot” filtration funnel (1), heated by steam, a chemical funnel (2) with a pleated filter (3), which is placed in it.
The hot, saturated solution of the substance is quickly poured onto a paper filter placed in a glass funnel, which is heated using a hot filter funnel. The filtrate is collected in a beaker or conical flask. When substance crystals form on the filter, they are washed with a small amount of hot solvent.
Cooling the solution
When the filtrate is cooled to room temperature, the crystallization process begins. To speed it up, the filtrate is cooled under running water. cold water. In this case, the solubility of the substance decreases, and final crystallization occurs.
Separation of formed crystals
The separation of crystals from the solvent is carried out by filtration, while suction or creating a vacuum in the receiver is often used to speed up the filtration process. To do this, use a vacuum pump (water jet, oil or Kamovsky).
Filtration is carried out in an installation that consists of a Buchner funnel (1) with a paper filter, a Bunsen flask or a special test tube (2), an intermediate beaker (3) and a vacuum pump. The size of the paper filter must exactly match the area of the bottom of the Buchner funnel.
The paper filter is moistened with solvent, placed in a funnel and the vacuum pump is turned on. When the pump operates, a reduced pressure is created under the filter - a characteristic sound occurs, which indicates the presence of vacuum in the system and the possibility of filtration. The cooled crystalline product together with the solvent, while shaking, is transferred in small portions from the conical flask to a paper filter.
During the filtration process, the solvent passes through the filter and the precipitate remains on it. Care should be taken that the filtrate does NOT fill the flask to the level of the tube connected to the intermediate glass. Filtration is continued until the filtrate stops dripping. After this, the precipitate is squeezed out on the filter with a wide glass stopper or a special glass rod, the pump is turned off, the precipitate is washed with a clean solvent, the pump is turned on and squeezed out again. The installation is disconnected from the vacuum, the funnel is removed. The filter along with the substance is carefully transferred to a Petri dish or a special container for drying.
Drying the solid
The solid can be dried in air at room temperature. Hygroscopic substances are dried in desiccators; resistant to air and temperature - in a drying cabinet, where the temperature should be 20 - 50 ° C below the melting point of the substance. For the recrystallized and dried product, the mass, yield and melting point are determined.
Melting point determination
The melting point of a substance is the temperature interval from the beginning to the complete melting of this substance. The purer the substance, the shorter this interval. The difference between the temperature at which the formation of the liquid phase begins and the temperature of complete melting for pure compounds does not exceed 0.5°C.
The presence of a small amount of impurities in a substance reduces its melting point and accordingly increases the melting range. This property is used to establish the identity of two substances, if one of them is known: equal amounts of substances are thoroughly mixed and the melting point of the mixture is determined (mixed sample). If the melting point of the mixed sample is the same as that of the pure substance, it is concluded that both substances are identical.
The melting point of a crystalline organic substance is determined in a capillary. The capillary is removed from the glass tube by heating it on a burner flame. One end of the capillary is sealed.
The recrystallized substance is thoroughly ground on a watch glass or in a mortar. A small amount of the substance is collected with the open end of the capillary and thrown, sealed end down, into a glass tube ≈ 60 - 80 cm long, placed vertically on the laboratory table. The operation of filling the capillary is repeated several times until a solid column of substance 2 - 3 mm high is formed in it.
The filled capillary (1) is secured with rubber rings (2) on the thermometer (3) so that the sample of the substance is at the level of the thermometer balls. The heating of the device is adjusted so that the temperature increases at a rate of 1°C per minute. At the same time, they carefully monitor the state of the column of substance in the capillary, noting all changes - changes in color, decomposition, sintering, wetting, etc. The beginning of melting is considered to be the appearance of the first drop in the capillary (T 1), and the end is the end of melting of the last crystals of the substance ( T 2). The temperature range (T 2 - T 1) is called the melting point of a given substance (T pl).
Practical part
Cleaning Methods
1 way. 25 g of borax at 60 0 C are dissolved in 50 ml of water. The solution is quickly filtered through a pleated filter into a porcelain cup or glass cooled with snow. The filtrate is continuously stirred with a glass rod.
Sodium tetraborate precipitates in the form of small crystals, they are sucked off, washed with a small amount of cold water and recrystallization is repeated. The crystals are dried in air for 2 – 3 days. The resulting preparation has the formula Na 2 B 4 O 7 *10H 2 O and is suitable for setting the titer.
Method 2. 25 g of borax at 65 - 70 0 C are dissolved in 75 ml of water. The resulting solution is quickly filtered through a pleated filter inserted into a funnel with a cut end, or through a hot filter funnel. The filtrate is first cooled slowly to 25 - 30 0 C, and then quickly in ice water or snow, enhancing crystallization by stirring with a stick. The precipitated crystals are sucked off, washed with a small amount of ice water and dried between sheets of filter paper for 2 - 3 days. The dried borax crystals should easily come off the dry stick.
The percentage of practical yield of borax is calculated.
Recrystallized borax is stored in a jar with a well-ground stopper.
Methods for purifying substances vary and depend on the properties of the substances and their application. In chemical practice, the most common methods are: filtration, recrystallization, distillation, sublimation, salting out. Gas purification is usually carried out by absorbing gaseous impurities with substances that react with these impurities. Pure substances have their own characteristic physical and chemical properties. Therefore, the purity of a substance can be checked by both physical and chemical methods. In the first case, density, melting, boiling, freezing points, etc. are determined. Chemical testing methods are based on chemical reactions and are methods of qualitative analysis.
In accordance with the standard (GOST), according to the degree of purity, reagents are divided into:
a) chemically pure (reagent grade),
b) pure for analysis (analytical grade),
c) clean (h.) and others.
Substances marked with chemical grade are suitable for laboratory work in inorganic chemistry. and ch.d.a.
- Recrystallization
To recrystallize a substance, it is dissolved in distilled water or a suitable organic solvent at a certain temperature. A crystalline substance is introduced into a hot solvent in small portions until it stops dissolving, i.e. a solution saturated at a given temperature is formed. The hot solution is filtered using a hot filtration funnel. The filtrate is collected in a glass placed in a crystallizer with cold water with ice or a cooling mixture. When cooled, small crystals fall out of the filtered saturated solution, since the solution becomes supersaturated at a lower temperature. The precipitated crystals are filtered on a Buchner funnel, then transferred to a sheet of filter paper folded in half. Using a glass rod or spatula, distribute the crystals in an even layer, cover with another sheet of filter paper and squeeze the crystals between the sheets of filter paper. The operation will be repeated several times. Then the crystals are transferred to a bottle. The substance is brought to constant mass in an electric drying cabinet at a temperature of 100-105 . The temperature in the cabinet should be increased gradually to this limit. To obtain a very pure substance, recrystallization is repeated several times.
- Sublimation (sublimation)
- Distillation (distillation)
- a) at atmospheric pressure (simple distillation),
- b) at reduced pressure (vacuum distillation),
- c) steam distillation.
- Salting out
Salting out is that under the influence of significant quantities of a saturated solution of a strong electrolyte, high-molecular natural compounds (proteins, gums, mucus, pectins) precipitate from the extracts. This occurs because when an electrolyte solution is added to the extract, the resulting electrolyte ions are hydrated, removing water from the biopolymer molecules. The protective hydration layer of biopolymer molecules disappears. Particle aggregation and biopolymer deposition are observed. Salting out is quite widely used for the purification of protein drugs, such as pepsin. The term “salting out” gets its name from the process of precipitation of proteins when sodium chloride is added to their solutions.
It must be borne in mind that different salts have different salting out properties, which is explained by the ability of anions and cations to hydrate. The salting out ability of electrolytes depends mainly on anions. Anions, according to their salting out power, are arranged in the following lyotropic series >>>>>.
For cations there is the same lyotropic series: > > > > .
However, sodium chloride is usually used for this purpose, which is cheaper.
- Sodium chloride
Sodium chloride is a chemical compound NaCl, sodium salt of hydrochloric acid, sodium chloride.
Sodium chloride is known in everyday life as table salt, of which it is the main component. Sodium chloride is found in significant quantities in sea water, creating its salty taste. It occurs naturally in the form of the mineral halite (rock salt).
Pure sodium chloride appears as colorless crystals. But with various impurities, its color can take on a blue, purple, pink, yellow or gray tint.
Moderately soluble in water, solubility depends little on temperature: the solubility coefficient of NaCl (in g per 100 g of water) is 35.9 at 21 °C and 38.1 at 80 °C. The solubility of sodium chloride is significantly reduced in the presence of hydrogen chloride, sodium hydroxide, and salts - metal chlorides. Dissolves in liquid ammonia and enters into exchange reactions.
- Sodium chloride called "table salt"
Table salt (sodium chloride, NaCl; the names “sodium chloride”, “table salt”, “rock salt”, “edible salt” or simply “salt” are also used) is a food product. When ground it appears as small crystals white. Table salt of natural origin almost always contains admixtures of other mineral salts, which can give it shades of different colors (usually gray). Produced in different types: purified and unrefined (rock salt), coarse and finely ground, pure and iodized, sea salt, etc. Salt is obtained by industrial purification of halite (rock salt) deposits located on the site of dried up seas.
- Sodium chloride occurs naturally in the form of the mineral halite.
Halite (Greek ??? - salt) is rock salt, a mineral of the chloride subclass, a crystalline form of sodium chloride (NaCl). The raw material from which table salt is made. Halites can be found in layers of sedimentary rocks among other minerals - products of water evaporation - in drying estuaries, lakes, and seas. The sedimentary layer is up to 350 meters thick and extends over vast areas. For example, in America and Canada, underground salt deposits extend from the Appalachian Mountains west of New York through Ontario to the Michigan basin.
- Purification of sodium chloride by salting out method.
When recrystallizing substances whose solubility changes little with temperature, the salting out method is used. Substances are added to solutions of such substances to reduce their solubility.
- Experimental part
Equipment: technochemical scales, mortar, beaker, tile, folded and ordinary filters, beaker, glass rod, funnel, Petri dish.
Reagents: saturated sodium chloride solution, table salt, distilled water, concentrated hydrochloric acid (? = 1, 19 ) .
- Cleaning Method
- Conducting an experiment
I weighed out 20 g of table salt on a technochemical scale and poured it into a glass. Added 50 ml of distilled water there. Then she placed the glass on the stove and brought the contents to a boil. The salt has flaked off. I filtered the solution and placed it in a fume hood. There, slowly, while stirring, I began to add concentrated hydrochloric acid. At the same time, the solubility of the electrolyte decreases when another electrolyte with the same ion is introduced into the solution. With the introduction of chlorine ions Cl? into a saturated solution of sodium chloride NaCl(k) > +Cl? the equilibrium shifts to the left, resulting in the precipitation of salt crystals that do not contain impurities.
I waited until the solution cooled down. The cooled solution was filtered. The resulting crystals were placed in a Petri dish and left to dry.
After the crystals dried, I weighed them: m=5,200 g.
etc.................